Call: 708-425-9080
Thermal Conductivity at Low Temperatures, Part 1: Theory
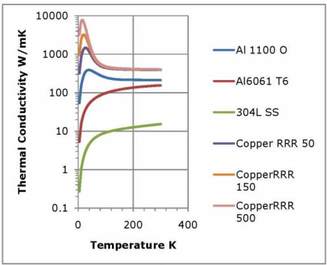 Figure 1. Thermal conductivities of selected metals as a function of temperature. Data from NIST.
Figure 1. Thermal conductivities of selected metals as a function of temperature. Data from NIST.
Thermal conduction is the process by which thermal energy is transported through matter. It is closely related to specific heat, the quantity of thermal energy contained in a substance. Both quantities are influenced by similar factors. One good source of data on materials at low temperatures is the NIST cryogenics group web page at http://cryogenics.nist.gov.
In metals, electrons are primarily responsible for conducting both heat and electricity. The contribution of the lattice to thermal conductivity in metals is small and is normally neglected. Impurities and lattice defects scatter electrons and decrease thermal conductivity. The highest thermal conductivities are obtained in very pure metals in their annealed state. Metals commonly encountered in low temperature work include stainless steel, aluminum, and copper. Metals typically have thermal conductivities in the range of 10 W/m-K (stainless steel alloys) to 400 W/m-K (copper) at room temperature. In many pure metals, and in most crystalline non-metals, the thermal conductivity increases as the temperature decreases until reaching a maximum value in the vicinity of 20K. This may seem surprising because the number of conduction electrons available to conduct heat falls with decreasing temperature. However, the number of phonons or lattice vibrations, which scatter electrons and limit conductivity, also decreases with temperature. The net effect is an increase in thermal conductivity with decreasing temperature until the temperature becomes so low that impurities and defects become the main limiting factor. At very low temperatures the thermal conductivity is proportional to temperature.
Particularly in copper, but in other metals as well, the thermal conductivity is a strong function of the purity and the condition of the metal. As a result, there is a considerable spread in the values of thermal conductivity reported in the literature, even for a specific alloy. To provide for better predictability, these metals are sometimes characterized by the residual resistance or RRR value. The RRR value is the ratio of the electrical resistivity at 4.2K to the electrical resistivity at 273K. This allows the thermal conductivity to be characterized, because there is a close correspondence between electrical and thermal conductivity in metals.
For cryogenic applications, copper and aluminum are used where good thermal conductivity is required, e.g. in heat exchangers and heat shields. Stainless steel is used in applications where a relatively poor thermal conductivity is suitable; e.g. in supports and structural members.
While good thermal conductivity and good electrical conductivity go hand in hand for normal metals, the reverse is true for superconductors. The Cooper pairs responsible for superconductivity do not take part in thermal conduction. Below the transition temperature, the thermal conductivity of a super conductor falls off rapidly. This must be kept in mind when using aluminum, which has a transition temperature of 1.2K, and when using some solder alloys.
In metals, electrons are primarily responsible for conducting both heat and electricity. The contribution of the lattice to thermal conductivity in metals is small and is normally neglected. Impurities and lattice defects scatter electrons and decrease thermal conductivity. The highest thermal conductivities are obtained in very pure metals in their annealed state. Metals commonly encountered in low temperature work include stainless steel, aluminum, and copper. Metals typically have thermal conductivities in the range of 10 W/m-K (stainless steel alloys) to 400 W/m-K (copper) at room temperature. In many pure metals, and in most crystalline non-metals, the thermal conductivity increases as the temperature decreases until reaching a maximum value in the vicinity of 20K. This may seem surprising because the number of conduction electrons available to conduct heat falls with decreasing temperature. However, the number of phonons or lattice vibrations, which scatter electrons and limit conductivity, also decreases with temperature. The net effect is an increase in thermal conductivity with decreasing temperature until the temperature becomes so low that impurities and defects become the main limiting factor. At very low temperatures the thermal conductivity is proportional to temperature.
Particularly in copper, but in other metals as well, the thermal conductivity is a strong function of the purity and the condition of the metal. As a result, there is a considerable spread in the values of thermal conductivity reported in the literature, even for a specific alloy. To provide for better predictability, these metals are sometimes characterized by the residual resistance or RRR value. The RRR value is the ratio of the electrical resistivity at 4.2K to the electrical resistivity at 273K. This allows the thermal conductivity to be characterized, because there is a close correspondence between electrical and thermal conductivity in metals.
For cryogenic applications, copper and aluminum are used where good thermal conductivity is required, e.g. in heat exchangers and heat shields. Stainless steel is used in applications where a relatively poor thermal conductivity is suitable; e.g. in supports and structural members.
While good thermal conductivity and good electrical conductivity go hand in hand for normal metals, the reverse is true for superconductors. The Cooper pairs responsible for superconductivity do not take part in thermal conduction. Below the transition temperature, the thermal conductivity of a super conductor falls off rapidly. This must be kept in mind when using aluminum, which has a transition temperature of 1.2K, and when using some solder alloys.
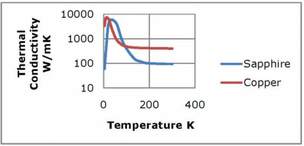 Figure 2. Thermal Conductivity of Sapphire and RRR 500 Copper
Figure 2. Thermal Conductivity of Sapphire and RRR 500 Copper
Nonmetals are often thought of as being relatively poor conductors of heat; howevercrystalline nonmetals can actually have very high thermal conductivities. The thermal conductivity of sapphire (aluminum oxide) actually exceeds that of very pure copper (RRR 500), from about 20K to 100K. Sapphire is used as an electrical insulator in heat sinking applications (magnet leads, for example) where excellent thermal conductivity and electrical isolation are required. The thermal conductivity of diamond reaches a peak value of 3000W/m-K at 80K, although it is not widely used due to its cost. Beryllium oxide and quartz are also examples of nonmetals that can exhibit relatively high thermal conductivities in crystalline form. In nonmetals, heat is conducted by lattice vibrations. In a good quality crystal that is free from defects and impurities, these lattice vibrations can travel over long distances and effectively transport heat. Lattice vibrations actually scatter in collisions with other lattice vibrations. Reducing the temperature reduces the number of lattice vibrations, but allows those remaining to travel further, thereby increasing the thermal conductivity. As with metals, a point is reached where impurities and defects limit the thermal conductivity, resulting in a peak value in the range of 10K to 100K depending on the material.
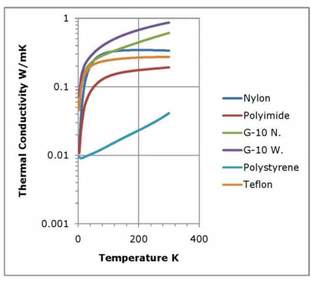 Figure 3. Thermal Conductivities of Selected Plastics. Data from NIST.
Figure 3. Thermal Conductivities of Selected Plastics. Data from NIST.
Plastics are widely used in cryogenic applications. Metalized plastics are used as insulation, plastic supports are used to minimize heat leaks and plastics are used to provide electrical isolation and to pot superconducting coils. Plastics have relatively low thermal conductivities; in the range of 0.2 W/m-K to 0.8 W/m-K at room temperature. Their thermal conductivities change very little until the temperature falls below 50K. At very low temperatures, the thermal conductivities of most nonmetals decrease as a function of T3.
The thermal conductivity of plastics may be modified with additives such as aluminum oxide. Thermal conduction in plastics, as in all nonmetals, is due to lattice vibrations. The lattice of amorphous materials lack the large-scale regularity of crystals, and lattice vibrations cannot propagate over large distances. Even plastics that are considered to be crystalline, such as nylon, do not have the same degree of order that is found in crystals such as quartz or sapphire. As a consequence, plastics as a whole have very similar properties. The thermal conductivity of G10, widely used to make supports in cryogenic equipment due to its favorable mechanical properties, has a slightly different thermal conductivity normal to and in the plane of the filler.
The thermal conductivities of common solid materials can range over seven orders of magnitude at low temperatures. At the lowest temperatures, the thermal conductivities of solids become very small. However, at intermediate temperatures, the thermal conductivity of a single substance, particularly if it is very pure, can vary over several orders of magnitude. These variations must be taken into account when designing cryogenic equipment. In Part 2 we will discuss proper material selection.
The Engineering Department at Meyer Tool has the knowledge and experience in cryogenic equipment to successfully incorporate a range of materials to achieve your design goals. Put this expertise to work for you in your next project. If this article was of interest to you, drop us a line and let us know. Your feedback will help us determine what type of content you would like to see in our newsletter and posted to the website.
The thermal conductivity of plastics may be modified with additives such as aluminum oxide. Thermal conduction in plastics, as in all nonmetals, is due to lattice vibrations. The lattice of amorphous materials lack the large-scale regularity of crystals, and lattice vibrations cannot propagate over large distances. Even plastics that are considered to be crystalline, such as nylon, do not have the same degree of order that is found in crystals such as quartz or sapphire. As a consequence, plastics as a whole have very similar properties. The thermal conductivity of G10, widely used to make supports in cryogenic equipment due to its favorable mechanical properties, has a slightly different thermal conductivity normal to and in the plane of the filler.
The thermal conductivities of common solid materials can range over seven orders of magnitude at low temperatures. At the lowest temperatures, the thermal conductivities of solids become very small. However, at intermediate temperatures, the thermal conductivity of a single substance, particularly if it is very pure, can vary over several orders of magnitude. These variations must be taken into account when designing cryogenic equipment. In Part 2 we will discuss proper material selection.
The Engineering Department at Meyer Tool has the knowledge and experience in cryogenic equipment to successfully incorporate a range of materials to achieve your design goals. Put this expertise to work for you in your next project. If this article was of interest to you, drop us a line and let us know. Your feedback will help us determine what type of content you would like to see in our newsletter and posted to the website.



